 According to the American Heart Association, 2019 marked the 100th year that cardiovascular diseases (CVD) have been the major cause of death in the United States. Collectively CVD account for 1 in 3 deaths at an annual cost of > $300 billion.
According to the American Heart Association, 2019 marked the 100th year that cardiovascular diseases (CVD) have been the major cause of death in the United States. Collectively CVD account for 1 in 3 deaths at an annual cost of > $300 billion.
Atherosclerosis is the chronic inflammation of arteries that underlies CVD, and leads to acute life threatening outcomes such as heart attack and stroke. This process is exacerbated by a host of risk factors associated with diet, metabolism, and lifestyle that are increasing in prevalence (e.g. dyslipidemia, obesity, diabetes) and contribute to a systemic inflammatory state.
 The endothelial cells that line the arteries are uniformly exposed to circulating inflammatory mediators elevated by these conditions. However, atherosclerosis progresses preferentially at sites of flow disturbance in arteries. This focal nature of atherosclerotic lesions implicates spatial heterogeneity in the regulation of inflammation by the endothelium.
The endothelial cells that line the arteries are uniformly exposed to circulating inflammatory mediators elevated by these conditions. However, atherosclerosis progresses preferentially at sites of flow disturbance in arteries. This focal nature of atherosclerotic lesions implicates spatial heterogeneity in the regulation of inflammation by the endothelium.
Fluid shear stress has been established as a determinant of the endothelial inflammatory phenotype. However, the mechanisms by which shear stress-mediated mechanotransduction enhances or suppresses the metabolic axis of inflammation to influence focal atherogenesis are not well understood.
Our studies are guided by overarching questions such as:
 How can we explain the non-random distribution of atherosclerosis within arteries? How does signaling via hemodynamic cues converge with a multitude of other factors driven by diet and metabolism to sensitize the endothelium to pathological change underlying atherosclerosis?
How can we explain the non-random distribution of atherosclerosis within arteries? How does signaling via hemodynamic cues converge with a multitude of other factors driven by diet and metabolism to sensitize the endothelium to pathological change underlying atherosclerosis?
Why do some individuals with the clinical risk factors for CVD never have a heart attack, while other apparently healthy individuals do? Why do treatments like statins only prevent heart attacks in some people?
Can we better predict an individual’s risk of having a heart attack by directly measuring markers of inflammation early in the process of atherosclerosis? Using these measures can we assess the effects of interventions such as diet, lifestyle change, or therapeutics?
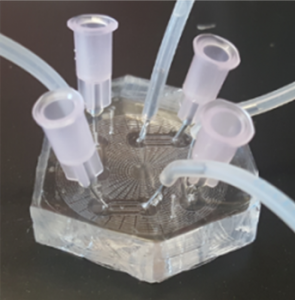 We employ artery-on-a-chip microfluidic tools in ex vivo models to study spatial and temporal inflammatory responses in endothelial cells under defined hydrodynamic conditions that mimic sites of susceptibility to atherosclerosis in arteries. We have applied these tools along with primary samples from the blood of human volunteers to study how shear stress modulates cytokine-, lipid-, and diabetes-induced inflammation, quantifying changes in endothelial cells and monocytes that mark among the earliest changes that promote atherosclerosis. One application of this approach has been to gauge an individual’s metabolic response to a meal high in saturated fat by quantifying the inflammatory capacity of their lipoproteins to endothelium and the capture efficiency of their circulating monocytes.
We employ artery-on-a-chip microfluidic tools in ex vivo models to study spatial and temporal inflammatory responses in endothelial cells under defined hydrodynamic conditions that mimic sites of susceptibility to atherosclerosis in arteries. We have applied these tools along with primary samples from the blood of human volunteers to study how shear stress modulates cytokine-, lipid-, and diabetes-induced inflammation, quantifying changes in endothelial cells and monocytes that mark among the earliest changes that promote atherosclerosis. One application of this approach has been to gauge an individual’s metabolic response to a meal high in saturated fat by quantifying the inflammatory capacity of their lipoproteins to endothelium and the capture efficiency of their circulating monocytes.
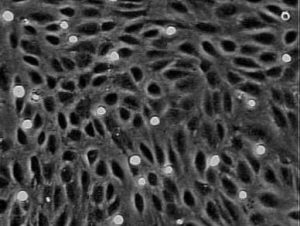 Collectively, our studies have contributed to the understanding of vulnerable endothelium and susceptibility to atherosclerosis. Our contributions have included:
Collectively, our studies have contributed to the understanding of vulnerable endothelium and susceptibility to atherosclerosis. Our contributions have included:
- Development of experimental tools and models for use in mechanistic studies of cultured endothelium, including microfluidic devices for the on-chip assessment of endothelial inflammatory phenotype.
- Elucidation of how disturbed blood flow is permissive to atherosclerosis, contributing to a state of chronic stress or dysregulation, which might be exacerbated by other inflammatory mediators elevated by traditional cardiometabolic risk factors.
 Enhanced understanding of how metabolic dysregulation of endothelium contributes to the earliest inflammatory changes that precede and promote atherosclerosis.
Enhanced understanding of how metabolic dysregulation of endothelium contributes to the earliest inflammatory changes that precede and promote atherosclerosis.- Elucidation of mechanisms by which mechanosignaling converges with metabolic factors to drive endothelial inflammation.
Please see our publications page for more detail.
